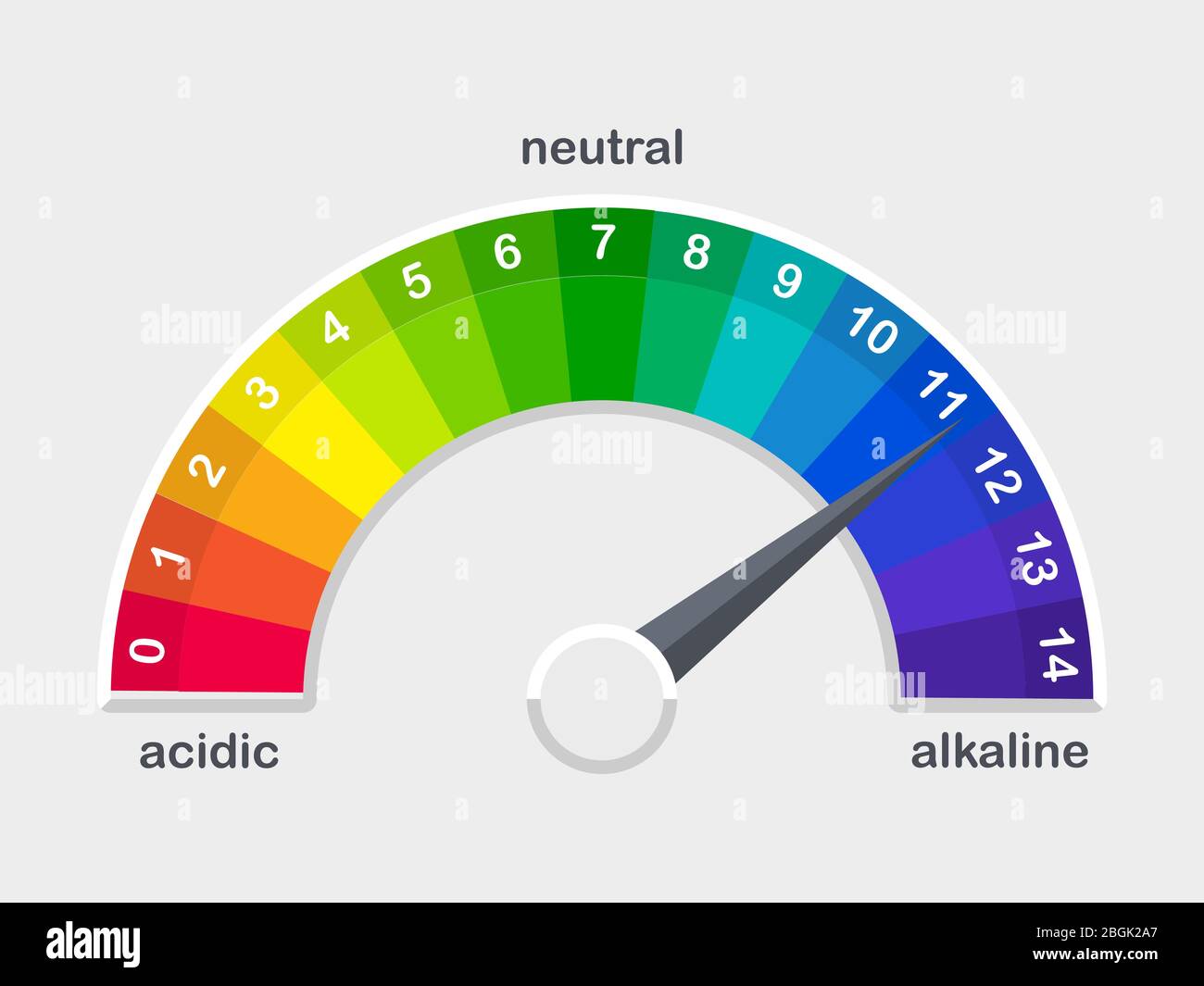
How To Measure Ph Of A Solution . ph represents the concentration of hydrogen ions (h+) in a solution and is measured on a logarithmic scale from 0 (strongly acidic) to 14. When pure water is dropped into a solution of. ph is defined as the negative log of hydrogen ion concentration. you can calculate the ph of a. the ph of an aqueous solution is the measure of how acidic or basic it is. The ph of an aqueous solution can be determined. to ensure accurate and consistent results, it is essential to use different measurement and maintenance practices. It can be used to describe the relative acidity (or basicity) of a solution. Alternatively, you can measure the activity of. the ph scale measures how strongly acidic or alkaline a solution is using a set of values from ph 0 to ph 14. to calculate the ph of a solution: Measure the concentration of hydrogen ion in the solution.
from www.alamy.com
Measure the concentration of hydrogen ion in the solution. to ensure accurate and consistent results, it is essential to use different measurement and maintenance practices. The ph of an aqueous solution can be determined. you can calculate the ph of a. Alternatively, you can measure the activity of. the ph of an aqueous solution is the measure of how acidic or basic it is. the ph scale measures how strongly acidic or alkaline a solution is using a set of values from ph 0 to ph 14. It can be used to describe the relative acidity (or basicity) of a solution. to calculate the ph of a solution: ph represents the concentration of hydrogen ions (h+) in a solution and is measured on a logarithmic scale from 0 (strongly acidic) to 14.
pH value colored scale meter for acid and alkaline solutions vector
How To Measure Ph Of A Solution When pure water is dropped into a solution of. The ph of an aqueous solution can be determined. ph is defined as the negative log of hydrogen ion concentration. to calculate the ph of a solution: When pure water is dropped into a solution of. the ph of an aqueous solution is the measure of how acidic or basic it is. Measure the concentration of hydrogen ion in the solution. to ensure accurate and consistent results, it is essential to use different measurement and maintenance practices. the ph scale measures how strongly acidic or alkaline a solution is using a set of values from ph 0 to ph 14. Alternatively, you can measure the activity of. you can calculate the ph of a. It can be used to describe the relative acidity (or basicity) of a solution. ph represents the concentration of hydrogen ions (h+) in a solution and is measured on a logarithmic scale from 0 (strongly acidic) to 14.
From www.expii.com
What Is pH? — Definition & Overview Expii How To Measure Ph Of A Solution to ensure accurate and consistent results, it is essential to use different measurement and maintenance practices. the ph of an aqueous solution is the measure of how acidic or basic it is. Measure the concentration of hydrogen ion in the solution. It can be used to describe the relative acidity (or basicity) of a solution. to calculate. How To Measure Ph Of A Solution.
From microbenotes.com
pH Meter Principle, Parts, Procedure, Types, Uses, Examples How To Measure Ph Of A Solution to calculate the ph of a solution: It can be used to describe the relative acidity (or basicity) of a solution. you can calculate the ph of a. ph is defined as the negative log of hydrogen ion concentration. to ensure accurate and consistent results, it is essential to use different measurement and maintenance practices. Measure. How To Measure Ph Of A Solution.
From alevelchemistry.co.uk
Acids Facts, Summary, Weak & Strong ALevel Chemistry Revision How To Measure Ph Of A Solution you can calculate the ph of a. to calculate the ph of a solution: Measure the concentration of hydrogen ion in the solution. It can be used to describe the relative acidity (or basicity) of a solution. The ph of an aqueous solution can be determined. When pure water is dropped into a solution of. the ph. How To Measure Ph Of A Solution.
From preparatorychemistry.com
pH and Equilibrium How To Measure Ph Of A Solution to calculate the ph of a solution: ph represents the concentration of hydrogen ions (h+) in a solution and is measured on a logarithmic scale from 0 (strongly acidic) to 14. When pure water is dropped into a solution of. ph is defined as the negative log of hydrogen ion concentration. the ph scale measures how. How To Measure Ph Of A Solution.
From www.youtube.com
Lab Equipment pH measurement with paper and meters [Part 1] YouTube How To Measure Ph Of A Solution The ph of an aqueous solution can be determined. When pure water is dropped into a solution of. ph represents the concentration of hydrogen ions (h+) in a solution and is measured on a logarithmic scale from 0 (strongly acidic) to 14. Measure the concentration of hydrogen ion in the solution. ph is defined as the negative log. How To Measure Ph Of A Solution.
From www.wikihow.com
3 Ways to Measure the pH of Water wikiHow How To Measure Ph Of A Solution to ensure accurate and consistent results, it is essential to use different measurement and maintenance practices. ph is defined as the negative log of hydrogen ion concentration. Measure the concentration of hydrogen ion in the solution. the ph of an aqueous solution is the measure of how acidic or basic it is. the ph scale measures. How To Measure Ph Of A Solution.
From www.britannica.com
PH Definition, Uses, & Facts Britannica How To Measure Ph Of A Solution When pure water is dropped into a solution of. It can be used to describe the relative acidity (or basicity) of a solution. Measure the concentration of hydrogen ion in the solution. The ph of an aqueous solution can be determined. to ensure accurate and consistent results, it is essential to use different measurement and maintenance practices. ph. How To Measure Ph Of A Solution.
From www.fierceelectronics.com
Eight Ways To Improve pH Measurement Reliability FierceElectronics How To Measure Ph Of A Solution to calculate the ph of a solution: Measure the concentration of hydrogen ion in the solution. When pure water is dropped into a solution of. The ph of an aqueous solution can be determined. the ph scale measures how strongly acidic or alkaline a solution is using a set of values from ph 0 to ph 14. . How To Measure Ph Of A Solution.
From study.com
The pH Scale Calculating the pH of a Solution Video & Lesson How To Measure Ph Of A Solution Alternatively, you can measure the activity of. to ensure accurate and consistent results, it is essential to use different measurement and maintenance practices. you can calculate the ph of a. the ph scale measures how strongly acidic or alkaline a solution is using a set of values from ph 0 to ph 14. to calculate the. How To Measure Ph Of A Solution.
From www.mometrix.com
pH Overview (Chemistry Review Video) How To Measure Ph Of A Solution Alternatively, you can measure the activity of. to calculate the ph of a solution: When pure water is dropped into a solution of. ph represents the concentration of hydrogen ions (h+) in a solution and is measured on a logarithmic scale from 0 (strongly acidic) to 14. to ensure accurate and consistent results, it is essential to. How To Measure Ph Of A Solution.
From naturalbiohealth.com
How Learning the pH Scale Can Create a More Balanced Diet Natural Bio How To Measure Ph Of A Solution It can be used to describe the relative acidity (or basicity) of a solution. ph represents the concentration of hydrogen ions (h+) in a solution and is measured on a logarithmic scale from 0 (strongly acidic) to 14. The ph of an aqueous solution can be determined. Alternatively, you can measure the activity of. ph is defined as. How To Measure Ph Of A Solution.
From www.linkedin.com
What is pH Scale? How to Measure pH Value Through pH Scale? How To Measure Ph Of A Solution the ph scale measures how strongly acidic or alkaline a solution is using a set of values from ph 0 to ph 14. to calculate the ph of a solution: It can be used to describe the relative acidity (or basicity) of a solution. the ph of an aqueous solution is the measure of how acidic or. How To Measure Ph Of A Solution.
From www.newagenutrients.com
pH Scale newagenutrients How To Measure Ph Of A Solution the ph scale measures how strongly acidic or alkaline a solution is using a set of values from ph 0 to ph 14. It can be used to describe the relative acidity (or basicity) of a solution. Measure the concentration of hydrogen ion in the solution. ph represents the concentration of hydrogen ions (h+) in a solution and. How To Measure Ph Of A Solution.
From exoesuwfw.blob.core.windows.net
What Does A Ph Meter Actually Measure at Robert Sparkman blog How To Measure Ph Of A Solution you can calculate the ph of a. Alternatively, you can measure the activity of. the ph of an aqueous solution is the measure of how acidic or basic it is. to ensure accurate and consistent results, it is essential to use different measurement and maintenance practices. When pure water is dropped into a solution of. the. How To Measure Ph Of A Solution.
From www.mt.com
pH Measurement of Organic Solvents METTLER TOLEDO How To Measure Ph Of A Solution ph is defined as the negative log of hydrogen ion concentration. you can calculate the ph of a. to calculate the ph of a solution: Alternatively, you can measure the activity of. to ensure accurate and consistent results, it is essential to use different measurement and maintenance practices. the ph of an aqueous solution is. How To Measure Ph Of A Solution.
From enggyd.blogspot.com
Engineers Guide pH Measuring Instruments, Working Principle, Theory How To Measure Ph Of A Solution Measure the concentration of hydrogen ion in the solution. to ensure accurate and consistent results, it is essential to use different measurement and maintenance practices. The ph of an aqueous solution can be determined. to calculate the ph of a solution: When pure water is dropped into a solution of. ph represents the concentration of hydrogen ions. How To Measure Ph Of A Solution.
From www.slideserve.com
PPT Chemistry 232 PowerPoint Presentation, free download ID3553045 How To Measure Ph Of A Solution ph is defined as the negative log of hydrogen ion concentration. When pure water is dropped into a solution of. you can calculate the ph of a. the ph of an aqueous solution is the measure of how acidic or basic it is. the ph scale measures how strongly acidic or alkaline a solution is using. How To Measure Ph Of A Solution.
From sciencenotes.org
How to Calculate pH Formula and Examples How To Measure Ph Of A Solution The ph of an aqueous solution can be determined. When pure water is dropped into a solution of. the ph of an aqueous solution is the measure of how acidic or basic it is. ph is defined as the negative log of hydrogen ion concentration. the ph scale measures how strongly acidic or alkaline a solution is. How To Measure Ph Of A Solution.